A common feature of many neurodegenerative diseases are pathological protein deposits in the brain. These protein aggregates cause nerve cells to die and, as a result, entire brain areas to shrink, which manifests in affected individuals as progressive dementia. The so-called tau protein in particular is involved in the development of neurodegenerative diseases such as Alzheimer’s and frontotemporal dementia.
A research team led by Professor Dr. Evgeni Ponimaskin, a scientist at the MHH Institute of Neurophysiology, has already discovered that signal transmission through a specific serotonin receptor called 5-HT7R plays a crucial role in this process.
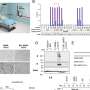
Now, in collaboration with international scientiststhe MHH team has investigated the effect of the antipsychotic amisulpride on the receptor. The drug, which is approved for the treatment of schizophrenia, can block the 5-HT7R and thus prevent the pathological accumulation of the tau protein. The effect of amisulpride has been successfully tested in various cellular models as well as in animal models of dementia. The results have now been published in the journal Alzheimer’s & Dementia.
Sustained basic activity stimulates protein deposition
Serotonin is a messenger substance that controls a number of vital processes, such as blood clotting, learning processes or the sleep-wake rhythm. Since it also influences our mood, it is known as the “happiness hormone.” The messenger substance mediates its effects by activating certain receptors that are bound to the cell membrane. These serotonin receptors occur in different variants and are increasingly found in brain regions that are affected in dementia.
For the receptor 5-HT7R, Professor Ponimaskin has already found a high basal activity in previous studies. “This means that the receptor is permanently active, even without serotonin binding to it,” explains the neurophysiologist. Through its high activity, 5-HT7R stimulates a chemical change in tau proteins that promotes pathological accumulation in the cell. However, the pathological overactivity can be stopped by using counterparts, so-called inverse agonists, to block the receptor’s signal transmission.
Antipsychotic as a possible therapy
The research team had already patented the substance class of inverse agonists for the treatment of dementia. However, the known inverse receptor agonists could so far only be used in the laboratory and not clinically. Therefore, in their current publication, the researchers carried out a broad pharmacological screening of already approved drugs and investigated whether they could influence the serotonin receptor as a “side effect,” so to speak.
“The antipsychotic amisulpride was found to be a potent inverse 5-HT7R receptor agonist,” notes Dr. Josephine Labus, who conducted this study with Professor Ponimaskin. It is true that dead nerve cells could not be repaired. However, in the early stages of the disease, the drug could stop dementia or even prevent it altogether. The therapeutic effect of amisulpride was also shown, among other things, in nerve cells differentiated from human stem cells with disease-relevant mutations.
“Now, in cooperation with the Neurological Clinic of the LMU Munich and the German Center for Neurodegenerative Diseases in Magdeburg, we are preparing a Phase II clinical trial to test the effect of amisulpride in the treatment of patients with dementia,” explains Professor Ponimaskin. The study is to start before the end of this year.
More information: Kathrin Jahreis et al, Amisulpride as a potential disease‐modifying drug in the treatment of tauopathies, Alzheimer’s & Dementia (2023). DOI: 10.1002/alz.13090
Provided by Hannover Medical School
Citation: Investigating a schizophrenia drug as a new therapy against dementia (2023, May 25) retrieved 5 July 2023 from https://medicalxpress.com/news/2023-05-schizophrenia-drug-therapy-dementia.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
Note: This article have been indexed to our site. We do not claim legitimacy, ownership or copyright of any of the content above. To see the article at original source Click Here