Browsing Tag
Cabotegravir
1 post
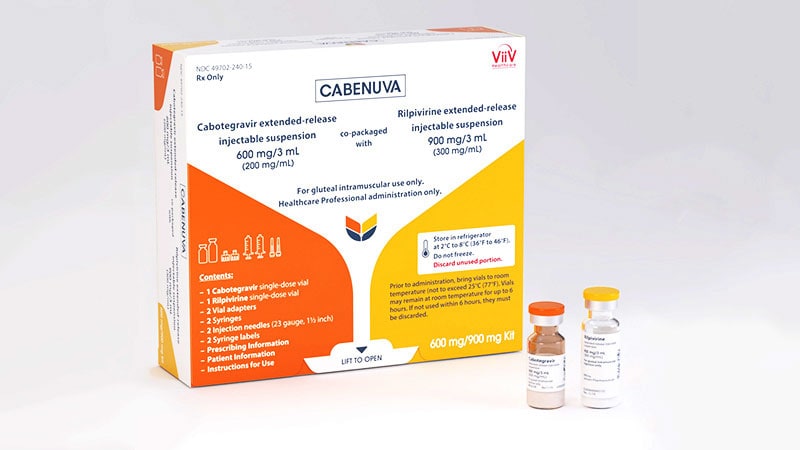
FDA Approves Cabotegravir LA for Long-Acting HIV PrEP
SILVER SPRING, MD — The FDA issued approval for extended-release injectable cabotegravir (Apretude, CAB-LA) today, providing an alternative to daily oral tenofovir disoproxil fumarate-emtricitabine (TDC-FTC) for pre-exposure prophylaxis (PrEP) against HIV acquisition. The priority review approval was based on phase 2b-3 clinical trial data submitted to the agency this past August, after one of the…
December 21, 2021