Users of at-home tests for COVID-19 may be less likely to follow CDC quarantine guidelines when using their testing kit instructions than with no instructions at all, a randomized trial found.
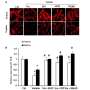
In a hypothetical involving a negative test result but a high risk of COVID exposure, 33% of participants who received the test’s FDA-authorized instructions were likely to fail to quarantine appropriately per CDC recommendations, as compared to 24% for a control group receiving no instructions (P=0.02) and 14% for an intervention group that received instructions based on “decision science principles” (P=0.004), reported Steven Woloshin, MD, of Dartmouth Institute for Health Policy and Clinical Practice in Lebanon, New Hampshire, and colleagues.
“These findings suggest that many at-home COVID-19 self-test users will draw false reassurance from a negative result, ignoring conditions that pose a high pretest probability of infection — and were perhaps the reason for testing,” they wrote in JAMA Internal Medicine.
For a scenario with a low risk of COVID exposure, 31% of those using authorized instructions were likely to quarantine unnecessarily following a negative test versus 10% with no instructions and 22% for the intervention (both non significant).
In all groups in all scenarios, “the proportion of incorrect responses was highest with the authorized instructions, even higher than with no instructions,” the authors wrote.
“The results of this study show how important it is to design and pilot-test instructions to ensure that they can be understood by as many users as possible,” they stated. “These findings also indicate that public health policies need to reflect both the limited sensitivity of rapid antigen tests and the possibility and probability of improper use.”
Woloshin’s group even suggested that authorized instructions were worse than no instructions at all, as they overrode “intuitive common sense — performance was poorer among this group than in the group with no instructions.”
The authors devised a survey to examine how people with varying COVID risks would respond to the results of a home test.
Participants were recruited in April 2021 for an online survey, and were paid $5. They were randomized to hypothetical scenarios involving an at-home rapid antigen COVID test, where they were asked to examine:
- FDA-authorized instructions (authorized)
- Instructions based in decision science principles that “emphasized clear, structured comparison of alternatives” (intervention)
- No instructions (control)
They were then further randomized to one of four hypothetical scenarios where they asked about a healthy unvaccinated individual, age 45, who had:
- COVID symptoms and recent close contact with someone who has COVID (“high pretest probability of infection”)
- No COVID symptoms and recent close contact
- COVID symptoms and no recent close contact
- No COVID symptoms and no recent close contact (“low pretest probability”)
The primary outcome was the proportion of participants “who failed to state” that the patient “should quarantine when appropriate, per CDC recommendations.”
Overall, 338 participants were included with a mean age of 38, and 54% were men. About two-thirds had a college degree; race/ethnicity information was not collected.
Not surprisingly, 95% of participants (95% CI 0.92-0.97) said they would quarantine appropriately given a positive test, regardless of which instruction group they were in.
Interestingly, 81%-82% of participants in both the authorized and intervention groups rated the instructions they were given as “easy or very easy to read,” while 94%-96% of both groups described the instructions as “useful or extremely useful” for interpreting home test results.
Limitations to the data include that it was based on hypothetical decisions in an online setting, and the self-selected sample that may limit generalizability of the findings.
“The potential benefits of at-home self-test kits will only be realized if users know how to interpret their results,” the authors wrote.
![author['full_name']](data:image/png;base64,R0lGODlhAQABAAD/ACwAAAAAAQABAAACADs=)
Molly Walker is deputy managing editor and covers infectious diseases for MedPage Today. She is a 2020 J2 Achievement Award winner for her COVID-19 coverage. Follow
Disclosures
The study was supported by the Swedish Foundation for Social Sciences and Humanities , the Agency for Healthcare Research and Quality Comparative Health System Performance Initiative, and the S&R Foundation’s Kuno Award for Applied Science for the Social Good.
Woloshin disclosed serving on editorial boards for the Cochrane Collaboration and JAMA Internal Medicine, as well as support from the National Cancer Institute and the State of New Mexico as an expert witness for a testosterone manufacturer for deceptive marketing.
Note: This article have been indexed to our site. We do not claim legitimacy, ownership or copyright of any of the content above. To see the article at original source Click Here



![author['full_name']](https://indexofnews.com/wp-content/uploads/sites/2/2022/02/localimages/mollyWalker_188.jpg6212d810aae13.jpg)